CLINICAL TRIAL

Prospective, multicenter, single-arm study to evaluate the safety and efficacy of the
Vanquish® Water Vapor Ablation System
for prostate cancer
Participating in VAPOR 2
Am I Eligible?
- If you are ≥50 years of age and have been diagnosed with intermediate-risk prostate cancer, your physician will review your eligibility with you to see if you can participate in the VAPOR 2 study
- If you are a candidate, you will be scheduled to complete a series of tests to further confirm your eligibility to participate
What is the Vanquish Water Vapor Ablation System?
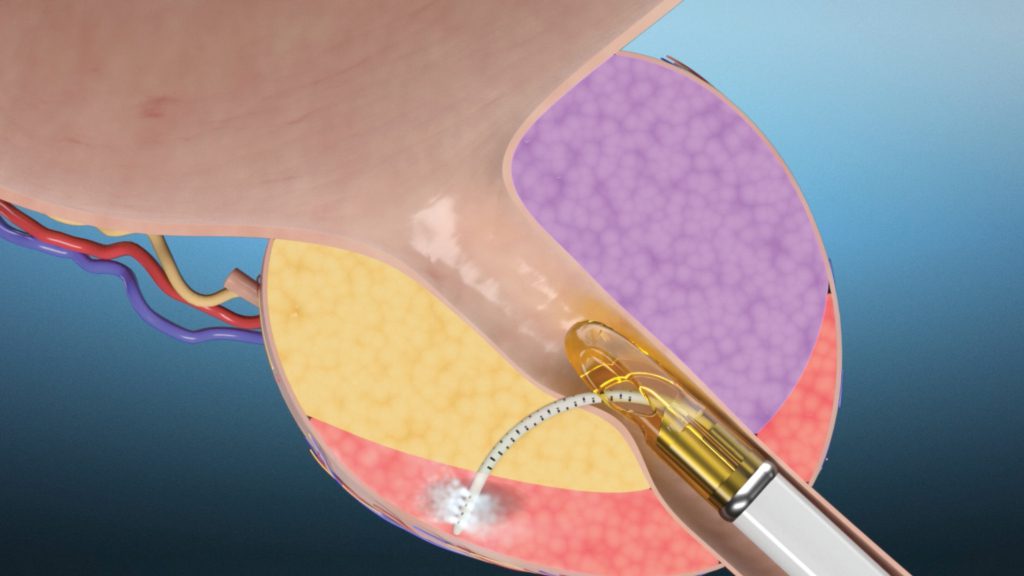
- The Vanquish Water Vapor Ablation System uses small amounts of water vapor (steam) to ablate (destroy) cancer cells
- The outpatient procedure is performed under general anesthesia
- A device is inserted into your urethra and advanced to your prostate. A small needle-shaped catheter is then deployed from the device and moved to the cancerous tissue. The physician will then deliver steam for 10 seconds to destroy the cancer cells
- Steam may also be delivered to areas around the cancerous tissue to ensure full ablation of the cancer

Understanding VAPOR 2
What Type of Study?
The Vapor 2 study identifies participants who will be treated with the Vanquish Water Vapor Ablation system and follows them for 5 years. The study will be conducted at multiple centers in the U.S.
What is being evaluated?
The VAPOR 2 study will evaluate whether the Vanquish system produces a reasonable assurance of safety and effectiveness for the ablation of prostate tissue and for the management of localized intermediate-risk prostate cancer.
Why should i participate in a research study?
The National Comprehensive Cancer Network (NCCN.org) encourages patients to participate in clinical trials and you may benefit as a result of your participation in this study. You will also receive close monitoring of your prostate cancer throughout the course of the study. There is, however, no guarantee that you will benefit from your participation in this study, but information learned from the study may help other prostate cancer patients in the future.
For more information, please visit clinicaltrials.gov/study/NCT05683691.
To explore whether you’re a good candidate, please send an email to vapor2@francismedical.com.

